The study uncovers a sharp increase in adverse-event reports filed with the FDA for gastroscopes and colonoscopes.
LFM Healthcare Solutions, LLC, has released its full report of U.S. Food & Drug Administration “MAUDE” data revealing that the number of medical device reports involving “patient-ready” flexible endoscopes that could have potentially exposed patients to infectious pathogens has risen sharply over the past eight years and continued to rise in 2021.
The study also notes that these “adverse-event reports” filed by hospitals, endoscope manufacturers, patients and other stakeholders rose markedly in all categories of studied endoscopes from 2014 to 2021 and continued to “increase significantly” from 2019 to 2020 for urological endoscopes and colonoscopes “when the number of elective or non-urgent endoscopic procedures performed during the pandemic decrease in the U.S. and globally ….”
“While endoscope procedures are generally quite safe, my research found a concerning trend, that patients continue to face the threat of being exposed to a superbug from an endoscope if it isn’t properly cleaned,” said Lawrence F Muscarella, PhD, President of LFM Healthcare Solutions, LLC, a Lansdale, PA-based independent infection prevention and quality improvement company. “Ineffective cleaning of flexible endoscopes is a public health worry that can significantly increase a patient’s risk of acquiring a healthcare-associated infection.”
“Superbugs” are organisms that are resistant to multiple types of antibiotics. Some superbugs today have developed resistance even to “last-resort” antibiotics, such as carbapenems.
Dr. Muscarella spent more than six months manually reviewing more than 10,000 adverse-event reports submitted to the FDA’s Manufacturer and User Facility Device Experience database, or MAUDE. Reports are submitted by manufacturers and healthcare facilities in accordance with mandatory federal requirements, and by voluntary reporters, including patients and consumers.
His detailed investigation, Contamination of Flexible Endoscopes and Associated Infections: A Comprehensive Review of Adverse-Event Reports Submitted to FDA, found that for all six of the studied endoscope types, the number of counted reports satisfying this analysis’s inclusion criteria – those FDA reports describing actual or potential contamination of a reprocessed endoscope — has increased significantly since 2014.
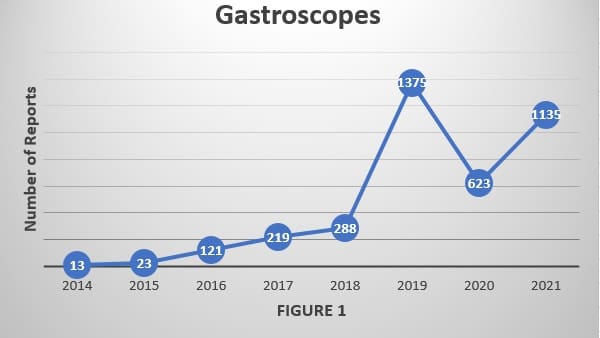
The increase was most marked for gastroscopes1,2; however, whose number of relevant reports rose more than 80 percent from 2020 to 2021 alone. The significant rise in adverse-event reports for gastroscopes, and similarly for colonoscopes too during this same timeframe, have not been directly addressed in a recent FDA safety communication, Dr. Muscarella said.
“Such an educational notice with specific safety recommendations would help to reduce the infection risk and bring attention to specific missteps that sometimes can be overlooked when resources are limited – for example, inadvertently failing to reprocess every potentially contaminated endoscope channel,” Dr. Muscarella added.
The FDA has recently published such safety communications for reducing infection risks around bronchoscopes and duodenoscopes warning of risks of superbugs infections and advising consideration of either sterilization or adopting single-use endoscopes, but FDA has not provided similar advice for gastroscopes, colonoscopes, or urological endoscopes. The number of relevant reports involving an ENT (“ear-nose-throat”) endoscope in 2021 also rose considerably compared to 2014, from 1 to 55.
The number of adverse-event reports for gastroscopes, which included ultrasonic models and echo–endoscope models, used to better visualize deep tissues and abnormalities, increased 82 percent to 1,135 in 2021, up from 623 in 2020. In 2014 there were just 13 gastroscope adverse reports satisfying his inclusion criteria, Dr. Muscarella found.
Meantime, adverse-event reports associated with colonoscopes rose 79 percent to 820 in 2021, up from 459 in 2020 and up from 23 in 2014. And urological scopes not only saw a marked increase in relevant adverse-event reports but also were connected to several bacterial outbreaks between 2015 and 2016 alone.
His report also uncovered that even after the FDA began offering guidance to hospitals in 2015 on enhancing cleaning of duodenoscopes there continue to be problems today. Adverse-event reports associated with “reprocessed” duodenoscopes increased to 651 in 2021, up almost 50 percent from 431 in 2020 and up from 72 in 2014.
“Duodenoscopes are still posing some of the same kinds of infection risks,” Dr. Muscarella said. “Due diligence is still required. This problem is not in our rearview mirror yet.” He also stresses, however, that healthcare staffers should take pride in their work knowing that the number of recorded deaths linked to a contaminated duodenoscope has decreased in the past few years.
Dr. Muscarella’s analysis is consistent with FDA guidance of “placing emphasis on the meticulous cleaning and either high-level disinfection or sterilization of flexible endoscopes ….” according to his report. In procedures that call for using a bronchoscope, Dr. Muscarella agrees with the FDA that consideration should be given to single-use scopes when, according to him, “the risk of transmitting multidrug-resistant microorganism is increased, or if the bronchoscope cannot be promptly reprocessed.”
Dr. Muscarella used 2014 as a starting point for the analysis because that was the year “U.S. health officials, for the first time, publicly linked a duodenoscope at one facility to multiple infections and deaths caused by superbugs called carbapenem-resistant Enterobacteriaceae (“CRE”) despite healthcare staffers reportedly having cleaned and disinfected the endoscope in compliance with manufacturer instructions,” he wrote.
“This outbreak marked a watershed moment at the time as virtually every previous case of infection linked to a contaminated flexible endoscope had been attributed to one or more specific reprocessing breaches (or a device design flaw),” according to his study.
Dr. Muscarella’s study was funded by Ambu Inc., the global leader in single-use endoscopes, but the author notes he maintained “complete and unfettered control over this study’s preparation, design, format displayed results, content and discussed limitations.”




























































